The earliest recorded woman in science was a woman of colour and one of the earliest known person in STEM at all.
Merit Ptah
("beloved of [the god] Ptah") lived circa 2700 BCE and was chief
physician of the pharoah's court, implying not only that she was
recognized as a doctor, who attended the pharoah, but that she trained
and supervised other doctors, during the Second or Third Dynasty of
Ancient Egypt.
 |
| Merit Ptah, Chief Physician, linocut by Ele Willoughby, 11" x 14", 2018 |
Hypatia, the first recorded female mathematician lived in the 3rd century AD in Alexandria, Egypt, which was part of the
Roman Empire. She was born at some time between about 350 and 370 and
died in 415 C.E. She was the head of the Platonist school, where she
taught philosophy, astronomy and mathematics. She believed in empiricism
and natural law. She was the last librarian of the famed Library of
Alexandria in the Museum of Alexandria, largest and most significant
library of the ancient world. She was the daughter of a famous
mathematician, Theon Alexandricus (ca. 335–405), with whom she worked
and published edited versions of Classical texts in mathematics. She
also pursued her education in Athens and Italy before returning to
Alexandria and becoming the head of the Platonist school. It is known that she wrote commentaries on 13-volume Arithmetica by
Diophantus, the Conics of Apollonius, and edited Ptolemy's Almagest and
on Euclid's Elements. She charted heavenly bodies. She built and instructed her pupils in the
design and use of the astrolabe, and likely made improvements to it.
 |
| Hypatia, linocut 12" x 12" by Ele Willoughby, 2012 |
Margaret Lucas Cavendish, Duchess of Newcastle-upon-Tyne
(1623 – 1673), 17th-century English aristocrat, philosopher, poet,
scientist, fiction-writer, and playwright shown with her imaginary
world from her strange science fiction novel 'The Blazing World' which she appended to her scientific treatise 'Observations upon Experimental Philosophy'.
Maria Sibylla Merian (1647-1717), was the leading
entomologist of her day, traveller and scientific illustrator. She is
shown complete with pomegranate branch and the life cycle of a morpho butterfly
from caterpillar, to chrysalis in its cocoon to butterfly, inspired by
her famous work '
Metamorphosis insectorum Surinamensium' - a process she
discovered then carefully documented and explained.
German-born
Caroline Herschel
(1750 – 1848), while overshadowed by her brother William
(who discovered Uranus, amongst his other astronomical
accomplishments), was a real pioneer as a woman in astronomy and made
her own important contributions. In fact, she became the first salaried
female scientist, when King George III hired her to assist her brother,
at a time when there were few professional scientists anywhere. Hers was
a real life sort of scientific Cinderella story; deemed
unmarriageable, since a childhood bout of typhus stunted her growth, her
mother thought she should train to be a servant but William managed to
rescue his younger sister from their mother's clutches, under the
pretext that she might have the voice to be a solo singer in Handel's
oratorios, as she too was a natural musician. Of course, he also wanted a
woman to manage his bachelor household. Meanwhile, he developed a real
passion for astronomy and soon, so did his sister. She discovered 11
nebulae (2 of which turned out to be galaxies) which
were previously unknown! She also found 8 or 9 comets, as well as making
and sharing observations of comets discovered by others. She worked to
complete and publish her brother's star charts after his death.
Marie-Anne Paulze Lavoisier (1758 – 1836) was the wife of Antoine Lavoisier, who is often referred to as the 'father' of modern chemistry,
without any reference to his wife. Marie-Anne became interested in her husband's scientific pursuits and soon
joined him in the lab. She received formal training in the field from
his friends and colleagues
Jean Baptiste Michel Bucquet
and Philippe Gingembre. Marie-Anne also famously hosted scientific salons with luminaries
of the day and was taught. Jacques Louis David painted his Portrait of Anoine-Laurent Lavoisier and
his wife in 1788. He also trained Marie-Anne Paulze in drawing and
engraving, allowing her to accurately illustrate their experiements. And
she most definitely appears in her own drawings and engravings
documenting their work.
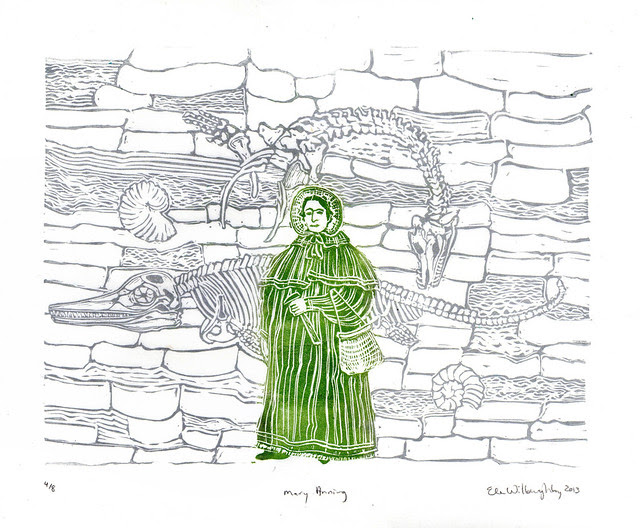
Mary Anning
(1799 – 1847) was the wrong class, the wrong
sex and even the wrong religious denomination to gain the education,
opportunity to work and communicate her results or to garner any respect
as a pioneering paleontologist. Further, during her lifetime most
people in Britain and elsewhere thought the Earth was a mere few
thousand years old, based on a very literal interpretation of the Bible
and found the idea of extinction did not fit in with the story of
creation. Yet, her fossil discoveries, meticulous collection,
documentation and independent work to fully understand the anatomy of
the amazing Jurassic creatures she encountered in famed Blue Lias cliffs
of Lyme Regis, Dorset, England, were so undeniable that she gained the
recognition, admiration and respect of the paleontologists of the day.
She made her first significant find, the first ichthyosaur skeleton to
be correctly identified, with her brother Joseph when she was only 12
years old. Her research showed that belemnite fossils contained
fossilised ink sacs
like those of modern cephalopods. She was also the first to recognize
that coprolites, known as bezoar stones at the time, were fossilised...
well, animal droppings (feces). While this sounds distinctly
unglamorous, the study of coprolites pioneered by Anning and Buckland
were vital to understanding ancient ecosystems. Her friend Henry De la
Beche painted the first widely circulated representation of a
prehistoric (deep time) scene, based on her finds, and he sold prints to
benefit her financially.
Anna Atkins (1799-1871),
née Children, was an English botanist and photographer. She is the first person to have illustrated a book using photographs,
Photographs of British Algae: Cyanotype Impressions in October 1843. Note that: not the first woman, the first
person.
She lived at a time when it was possible to be a self-trained
scientist, especially if you were middle or upper class and received an
education and the financial freedom to devote your time to pursue your
subject. She was raised and instructed by her father, a
naturalist, and her social circle included those who were developing (no
pun intended) the latest, brand new photographic technology. So, she
was at the right place at the right time. But that doesn't take away
from the fact that she had the knowledge, skill, insight and ability to
immediately see the utility of the method for descriptive science and to
document a specific field of sub-field of botany, with her collection
of the algae (seaweeds) of Britain. I think this should be understood as
equivalent to a modern-day scientist keeping abreast of other fields of
study and rapidly mastering a new high-tech tool to apply it to her
field. Even William Henry Fox Talbot, who who invented the salted paper
and calotype processes, precursors to modern photographic methods, was
not able to publish
The Pencil of Nature the first commercially printed photographic book, until eight months after she produced
Photographs of British Algae: Cyanotype Impressions.
Today is named in honour of
Countess, Lady Ada Lovelace (1815-1852), who published the first
computer program. She worked together with Charles Babbage, the inventor
of the Difference Engine and the Analytical Engine (the first -
analogue! - computers), correcting his notes on how to calculate
Bernoulli Numbers with the Analytical Engine. More importantly, she (a
great communicator, daughter of mad, bad and dangerous to know poet Lord
Byron) was able to understand and explain the workings of the
analytical engine and the potential of computing machines. Her comments
seem visionary to the modern reader. Babbage called her the Enchantress
of Numbers.
Founder of modern nursing, social reformer, statistician, data
visualization innovator and writer Florence Nightingale (1820 –
1910) earned the nickname "The Lady with the Lamp" during the Crimean War,
from a phrase used by The Times, describing her as a “ministering angel”
making her solitary rounds of the hospital at night with “a little lamp
in her hand”. Behind Nightingale is her own ‘Diagram of Causes of Mortality in the
Army in the East’ plotted as a polar area diagram – her own statistical
and data visualization innovation, sometimes called a Nightingale Rose
Diagram. It illustrates the causes of death in the military hospital she
managed during the Crimean War. When she researched
the causes of mortality, looking back at the data, she saw clearly that
the lack of hygiene was a far greater risk to soldiers’ lives than being
wounded. Her statistics and clear data visualization saved lives.
Great Russian mathematician and writer,
Sofia Vasilyevna Kovalevski
(1850-1891), is also known as Sofie
or Sonya, her last name has been transliterated from the Cyrillic Со́фья
Васи́льевна Ковале́вска in a variety of ways, including Kovalevskaya
and Kowalevski. Sofia's contributions to analysis, differential
equations and mechanics include the Cauchy-Kovalevski theorem and the
famed Kovalevski top (well, famed in certain circles, no pun intended).
We now know there are only three fully integrable cases of rigid body
motion and her solution ranks with those of mathematical luminaries
Euler and Lagrange.
She was the first woman appointed to a full professorship in Northern
Europe or to serve as editor of a major scientific journal. She is also
remembered for her contributions to Russian literature. All of this
despite living when women were still barred from attending university.
Her accomplishments were tremendous in her short but astonishing life.
My portrait of
Marie Skłodowska-Curie
(1867 – 1934) shows the famous
Polish-born, naturalized-French physicist and chemist at work in her
lab. The contents of her lab glassware appropriately glow-in-the-dark!
She was one of the pioneers who helped explain radioactivity, a term she
coined. She was the one who first developed a means of isolating
radioactive isotopes and discovered not one, but two new elements:
polonium (named for her native country) and radium. She also pioneered
radioactive medicine, proposing the treatment of tumors with
radioactivity. She founded medical research centres, the Curie
Institutes in Paris and Warsaw which are still active today. She created
the first field radiology centres during World War I. Marie Curie was
the first woman to win a Nobel prize, the only woman to
ever win TWO Nobel prizes, and the only person ever to win in two
different sciences: physics and chemistry!
Henrietta Swan Leavitt (1868- 1921) was an American astronomer. In her
day, women scientists were regularly hired to do menial chores. She was
hired to count images on photographic plates as a "computer". In
studying these plates,
in 1908 she was able to deduce a ground-breaking theory, which allowed Hubble's later insight about the age and expansion of the universe. Her period-luminosity relation for
Cepheid variable stars radically changed modern astronomy, an accomplishment for which she received little recognition during her lifetime.
Canadian medical researcher
Maud Menten
(1870-1960) has been called the "grandmother of biochemistry" and "a
radical feminist 1920s flapper," and a "petite dynamo." Not only was
she an author of Michaelis-Menten equation for enzyme kinetics (like
the plot in indigo in my portrait), she invented the azo-dye coupling
for alkaline phosphatase, the first example of enzyme histochemistry,
still used in histochemistry imaging of tissues today (which inspired
the histology background of the portrait), and she also performed the
first electrophoretic separation of blood haemoglobin in 1944!
 |
| Maud Menten, linocut 9.25" x 12.5" by Ele Willoughby, 2018 |
Physicist
Harriet Brooks
(1876 - 1933) shows her and her discovery of atomic recoil. Brooks
also discovered Radon and measured its atomic mass and half-life. Her
graduate supervisor and future Nobel laureate Ernest Rutherford also
credited her with first recognizing that radioactive elements could
undergo chains of transmutations into a series of new elements. He
stated that she was second only to Marie Curie in her capacity for and
ability as a radioactivity researcher. During her extraordinary 6 year
career in physics she worked with 3 Nobel laureates (Rutherford,
Thomson and Curie) and made these fundamental contributions to the new
field of nuclear physics!
Physicist
Lise Meitner (1878 – 1968) was the first person to provide a theoretical explanation for nuclear
fission and was an integral member of the experimental team as well (she collaborated with ollaborated with chemists
Otto Hahn and
Fritz Straßmann),
though her gender and her heritage interfered with her being properly
acknowledged in late 30s Germany. Only Hahn was awarded the Nobel for
this work. She received the Max Planck Medal of the German Physics
Society in 1949.
Meitner was nominated to receive the Nobel prize three times. In 1966
Hahn, Fritz Straßmann and Meitner together were awarded the Enrico Fermi
Award. In 1997, the element 109 was named meitnerium in her honour.
Today the Hahn-Meitner Institut in Berlin, craters on the Moon and on
Venus, and a main-belt asteroid are all named in her honour.
 |
| Lise Meitner (1878 – 1968) and Nuclear Fission, linocut by Ele Willoughby |
Geologist and paleontologist
Alice Wilson
(1881-1964) mapped the entire Ottawa-St Larence Valley region by
herself, since she was barred from doing fieldwork with men, was the
first female Canadian geologist, despite ill-health and a frail
constitution. Her research interests focused
on fossil invertebrates from the Paleozoic era (252–541 million years
ago) from across Canada, and from the Ordovician era (444–485 million
years ago) in her own backyard in Ontario and Quebec as well as
Ordovician fauna from the Rockies and Arctic. She studied stratigraphy
in Ontario and Quebec. Over the course of 50 years, she became an
authority on fossils and rocks of the Ottawa - St. Lawrence Valley, as a
direct response to the sexist limitations placed upon her.
 |
Alice Wilson, linocut on collaged washi papers, 11" x 14" by Ele Willoughby, 2018 |
Emmy Noether (1882-1935) was one of the greatest 20th century mathematicians and
Noether's Theorem is one of the most fundamental and profound theories in physics.
Inge Lehmann (1888-1993) was a pioneer woman in science, a
brilliant seismologist and lived to be 104. In 1936 she wrote an
earth-shattering paper, with an astonishingly succinct title: P' in
which she laid out her arguments supporting her discovery of the
inner core of the earth.
She later also discovered a discontinuity in the mantle (confusingly
called the Lehmann discontinuity). When she received the Bowie medal in
1971 (she was the first woman to
receive the highest honour of the American Geophysical Union), her
citation noted that the "
Lehmann discontinuity was discovered through
exacting scrutiny of seismic records by a master of a black art for
which no amount of computerization is likely to be a complete
substitute..."
 |
| Inge Lehmann, linocut on Japanese washi, 8" x 8" by Ele Willoughby |
Alice Augusta Ball
(1892 - 1916) was a chemist who discovered the first effective
treatment for leprosy (or Hansen's disease) a disfiguring disease which
has afflicted people for millenia. Physician Dr. Harry T. Hollmann of
the Kalihi Hospital in Hawai'i and
acting director of the Kalihi leprosy clinic, was unsatisfied with using
chaulmoogra oil in its natural form to treat leprosy patients and
wanted to isolate the active ingredients. He recruited the graduate
student Ball to help. Within a year, she was able to do what chemists
and pharmacologists had been unable to do for centuries. She not only
isolated the active ingredients but convert them to a form which could
be circulated in the body. My print shows how she formed the ethyl ester
of chaulmoogric acid (the acid plus alcohol produces the ethyl ester
with water).
 |
Alice Ball, 11" x 14", linocut by Ele Willoughby, 2018, shows the chemist, branches of the chaulmoogra tree and
how she formed the ethyl ester of chaulmoogric acid (the acid plus alcohol produces the ethyl ester with water) |
Mary Golda Ross (1908-2008) was a mathematician, aeronautical engineer, philanthropist and Cherokee “hidden figure” of the space race. Lockheed Martin hired her as mathematician in 1942, troubleshooting the
P-38 Lighting fighter plane (as shown). She knew already that her
interest was in interplanetary flight, but didn’t mention it in 1942 for
fear that her credibility would be questioned, but she was indeed
farsighted. After the war Lockheed Martin sent her to UCLA to study
engineering and celestial mechanics. She was one of the 40 engineers
selected to start Skunk Works, their Advanced Development Program, an
in-house top-secret think tank. She was the only woman and only
Indigenous person and much of her work there remains classified! It
included preliminary design concepts for interplanetary travel, crewed
and uncrewed space flights and the earliest plans for orbiting
satellites. She worked on the Agena rocket, so important to the Apollo
moon mission (shown) and was an author of the NASA Flight Handbook Vol.
III about flight to Mars and Venus.
 |
| Mary Golda Ross, linocut handprinted on Japanese kozo paper, 11" x 14", 2018 by Ele Willoughby |
|
|
Physicist
Chien-Shiung Wu
(1912-1997, Chinese-born American physicist, whose nicknames included
the “First Lady of Physics”, “Chinese Marie Curie,” and “Madame Wu”)
came up with a truly beautiful experiment to test whether the weak force
conserves parity. For their theoretical work on the question of parity in the physics of
subatomic particles, Lee and Yang were quickly awarded the Nobel Prize
for Physics in 1957; the Nobel committee neglected to include Wu.
Hedy Lamarr
(1914 – 2000), best known as a star of Hollywood's Golden Age was born
Hedwig Keisler, escaped Austria during WWII and her arms-dealer husband
and put her inside knowledge to work for the Allied forces. She knew
that torpedoes were guided by radio signals, of a single
frequency, which were vulnerable to interference or "jamming". She had
the idea that if multiple frequencies were employed, like a radio
station which varied its channel unpredictably, it would not be possible
for the enemy to find and interfere with the signal. This way the
signal could be encoded across a broad spectrum. She met her neighbour,
the avant-guard musician and composer George Antheil at a party.
Together they developed Hedy's frequency-hopping idea, encorporating
George's technology for synchronizing pianolas, and on the 11th of
August, 1942, US Patent Number 2,292,387 for the "Secret Communications
System." Lamarr's and Antheil's frequency-hopping idea serves as a basis
for
modern spread-spectrum communication technology, such as Bluetooth,
COFDM (used in Wi-Fi network connections), CDMA (used in some cordless
and wireless telephones) and 4G LTE communications. You are probably
using a device right now which relies on these ideas.
Irene Ayako Uchida
(1917-2013) was a geneticist and cytologist who discovered the risk
posed to future offspring due to abdominal x-rays on their pregnant
mothers. She was a world expert in Down syndrome, President of the
American Society of Human Genetics, served on the Science Council of
Canada, received honourary degrees from McMaster and Western
universities, was named Woman of the Century 1867-1967 by the National
Council of Jewish Women, in Manitoba, an Officer of the Order of Canada,
had a lifelong love of language and grammar, and a wry sense of humour.
 |
Irene Ayako Uchida, Linocut, 9.25" x 12.5", 2018 by Ele Willoughby
My
linocut portrait of Canadian geneticist Irene Ayako Uchida (1917-2013)
is hand printed on 9.25" x 12.5" Japanese kozo (or mulberry) paper.
Uchida is shown surrounded by chromosones, with anomalies (shown with
pink arrows) due to radiation exposure, based on one of her research
papers. A strand of DNA is hidden in the image (as her watchband). |
|
|
|
American geologist and oceanographic cartographer
Marie Tharp
(1920-2006), made pioneering, thorough and complete ocean floor maps
made with her partner in science Bruce Heezen which revealed the
Mid-Atlantic
Ridge. The mid-ocean ridge itself, based on their 1957 physiographic
map, is illustrated behind her, along with the sort of echo sounder or
precision depth recorder tracks she used, in front of her. This work
was integral to the Plate Tectonics revolution in earth science.
Beatrice "Trixie" Helen Worsley
(1921-1972) is believed to have earned the very first doctorate in
computer science, supervised by Douglas Hartree and Alan Turing at
Cambridge, set the WWII Wrens' record for time at sea, at 150 days, and
was the first female computer scientist in Canada.
 |
| Trixie Worsley, linocut 11" x 14" by Ele Willoughby, 2018 |
Ursula Franklin (1921 – 2016) represented not only excellence in science and engineering, but she was a great,
perhaps even visionary, thinker on the very role of technology in our
society, as well as a fearless and tireless advocate for women in STEM,
peace and social justice. Her research interests and achievements were
clearly guided by her principles, including gathering evidence of the
harmful health effects of radiation from atmospheric testing of nuclear
weapons to or her work on the political and societal impacts of support
of the technologies and their use. She was also a pioneer in archeometry: the application of material science to archeology.
Astrophysicist
Jocelyn Bell Burnell (born 1943) was just a graduate student in 1967 when she discovered the first radio pulsar
(or pulsating star), a highly magnetized, rotating neutron star that
emits a beam of electromagnetic radiation. This radiation (light in the
radio frequency band) can only be observed when the star is point
towards us; so, like the light from a distant lighthouse, it appears to
pulse at a precise frequency. The 1968 paper announcing this discovery in Nature has five authors,
lead by Hewish, followed by Jocelyn Bell. In 1974, Hewish won the Nobel
Prize for this discovery, along with fellow radioastronomer Martin
Ryle). Jocelyn Bell was not included as it was assumed that the "senior
man" was responsible for the work. Jocelyn Bell Burnell has gone one to a very distinguished career in
astrophysics. She became the first female president of the Institude of
Physics and of the Royal Society of Edinburg. She helped set up the
Athena Swan programme to support UK women in science. In 2018 she was
awarded the $3 million Breakthrough Prize in Fundamental Physics for her
discovery of pulsars and lifetime of leadership in science. She is
donating the award money to the Institute of Physics for PhD scolarships
for underrepresented people including women, ethnic minorities and
refugee students in physics!
Mae Carol Jemison
(born October 17, 1956) is a physician who became the first African
American woman to travel in space when she went into orbit aboard the
Space Shuttle Endeavour for NASA, on September 12, 1992. She also has a
B.S. in chemical engineering, served in the Peace Corps, is a dancer and
choreographer, formed and runs her own company researching the
application of technology to daily life, and even appeared on Star Trek:
The Next Generation.
 |
| Mae Jemison, linocut on Japanese kozo paper, 9.25" by 12.5" (23.5 cm by 32 cm) in an edition of eight by Ele Willoughby, 2014 |

 The first show for me this year was The Tarot Lovers: Works of Heart exhibition, which showed (and sold) at the Wellington County Museum and Archives. Curated by Shelley Carter, this show is one in a series she has organized with themes based on Tarot cards. I enjoyed the challenge of mixing those concepts with my ongoing works about the history of science, as an excuse to highlight the loving partnership of Antoine Lavoisier (26 August 1743 – 8 May 1794) and his wife Marie-Anne Paulze Lavoisier (20 January 1758 – 10 February 1836). Lavoisier is often referred to as the 'father' of modern chemistry, without any reference to his wife, and yet, as their official illustrator, she shows herself participating in his experiments and her skills as technical scientific translator allowed him to be up-to-date with chemistry across the Channel.
The first show for me this year was The Tarot Lovers: Works of Heart exhibition, which showed (and sold) at the Wellington County Museum and Archives. Curated by Shelley Carter, this show is one in a series she has organized with themes based on Tarot cards. I enjoyed the challenge of mixing those concepts with my ongoing works about the history of science, as an excuse to highlight the loving partnership of Antoine Lavoisier (26 August 1743 – 8 May 1794) and his wife Marie-Anne Paulze Lavoisier (20 January 1758 – 10 February 1836). Lavoisier is often referred to as the 'father' of modern chemistry, without any reference to his wife, and yet, as their official illustrator, she shows herself participating in his experiments and her skills as technical scientific translator allowed him to be up-to-date with chemistry across the Channel. The works, along with my previously exhibited 'Imaginary Menagerie' prints were exhibited at Balzac's upstairs gallery for the Curious Fauna show in the fall, and I got my very own wunderkammer of beautiful and very varied artist-made matchboxes on the theme of cabinets of curiosities. This delightful project was curated and created by Hearyung Kim and Natalie Draz (whom I know from PROOF Studio Gallery).
The works, along with my previously exhibited 'Imaginary Menagerie' prints were exhibited at Balzac's upstairs gallery for the Curious Fauna show in the fall, and I got my very own wunderkammer of beautiful and very varied artist-made matchboxes on the theme of cabinets of curiosities. This delightful project was curated and created by Hearyung Kim and Natalie Draz (whom I know from PROOF Studio Gallery).
 This August, the Ontario Science Centre (OSC) staged an exhibit called 'Quantum' about the history of quatum mechanics. My friends at The Maker Bean (where you can now find my art, at both their Bloor and Dufferin and OSC locations) asked if I had an artwork about quatum mechanics. Indeed I do! So my portraits of physicists Bohr, Meitner, Roentgen, Wu and Curie, as well as Schroedinger's Cat, have all been on display at the OSC since then!
This August, the Ontario Science Centre (OSC) staged an exhibit called 'Quantum' about the history of quatum mechanics. My friends at The Maker Bean (where you can now find my art, at both their Bloor and Dufferin and OSC locations) asked if I had an artwork about quatum mechanics. Indeed I do! So my portraits of physicists Bohr, Meitner, Roentgen, Wu and Curie, as well as Schroedinger's Cat, have all been on display at the OSC since then!  This August, the Ontario Science Centre (OSC) staged an exhibit called 'Quantum' about the history of quatum mechanics. My friends at The Maker Bean (where you can now find my art, at both their Bloor and Dufferin and OSC locations) asked if I had an artwork about quatum mechanics. Indeed I do! So my portraits of physicists Bohr, Meitner, Roentgen, Wu and Curie, as well as Schroedinger's Cat, have all been on display at the OSC since then!
This August, the Ontario Science Centre (OSC) staged an exhibit called 'Quantum' about the history of quatum mechanics. My friends at The Maker Bean (where you can now find my art, at both their Bloor and Dufferin and OSC locations) asked if I had an artwork about quatum mechanics. Indeed I do! So my portraits of physicists Bohr, Meitner, Roentgen, Wu and Curie, as well as Schroedinger's Cat, have all been on display at the OSC since then!